Connect with us
Published
2 months agoon
By
admin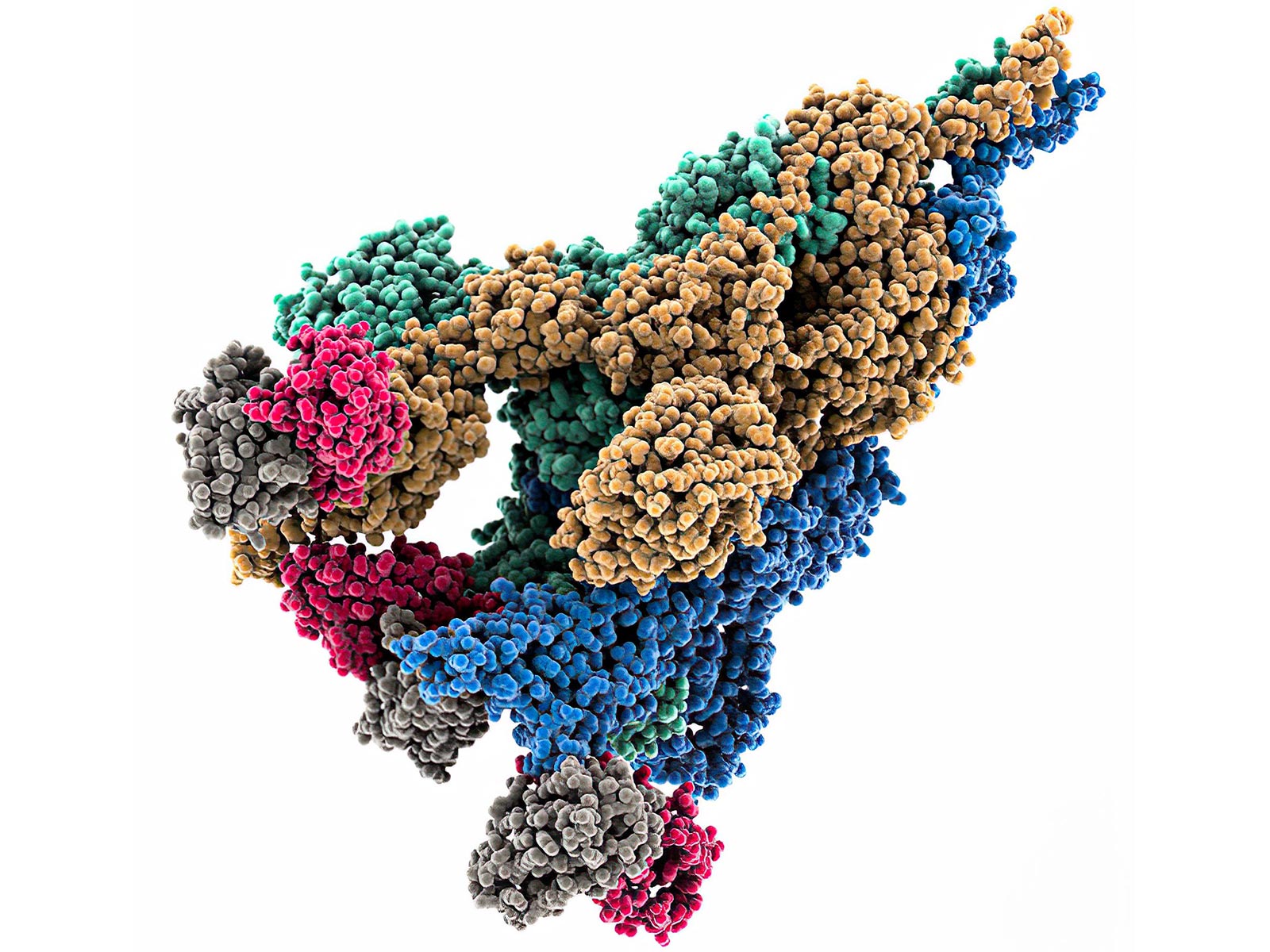
Researchers at RIKEN have identified how the D614G mutation in the SARS-CoV-2 virus enhanced its infectivity and transmission speed. This mutation alters the spike protein’s shape, enabling the virus to adapt more effectively to human hosts. The study utilized advanced molecular modeling, including simulations conducted on the RIKEN Fugaku supercomputer, to analyze the protein structure’s changes caused by the mutation. Specifically, the D614G mutation disrupts an ionic bond within the spike protein, leading to a conformational change that improves the virus’s ability to infect cells. This early mutation has had a significant global impact, becoming a characteristic feature of subsequent variants that emerged throughout the COVID-19 pandemic, including Alpha, Delta, and Omicron. The findings underscore the importance of even minor genetic changes in viral evolution, demonstrating how a single amino acid substitution can dramatically influence viral properties. As a result, this research may provide valuable insights for developing next-generation vaccines and antiviral treatments aimed at combating COVID-19 and its variants. The team is now investigating other adaptive mutations that occurred later in the pandemic to further enhance therapeutic strategies.

















