Connect with us
Published
2 weeks agoon
By
admin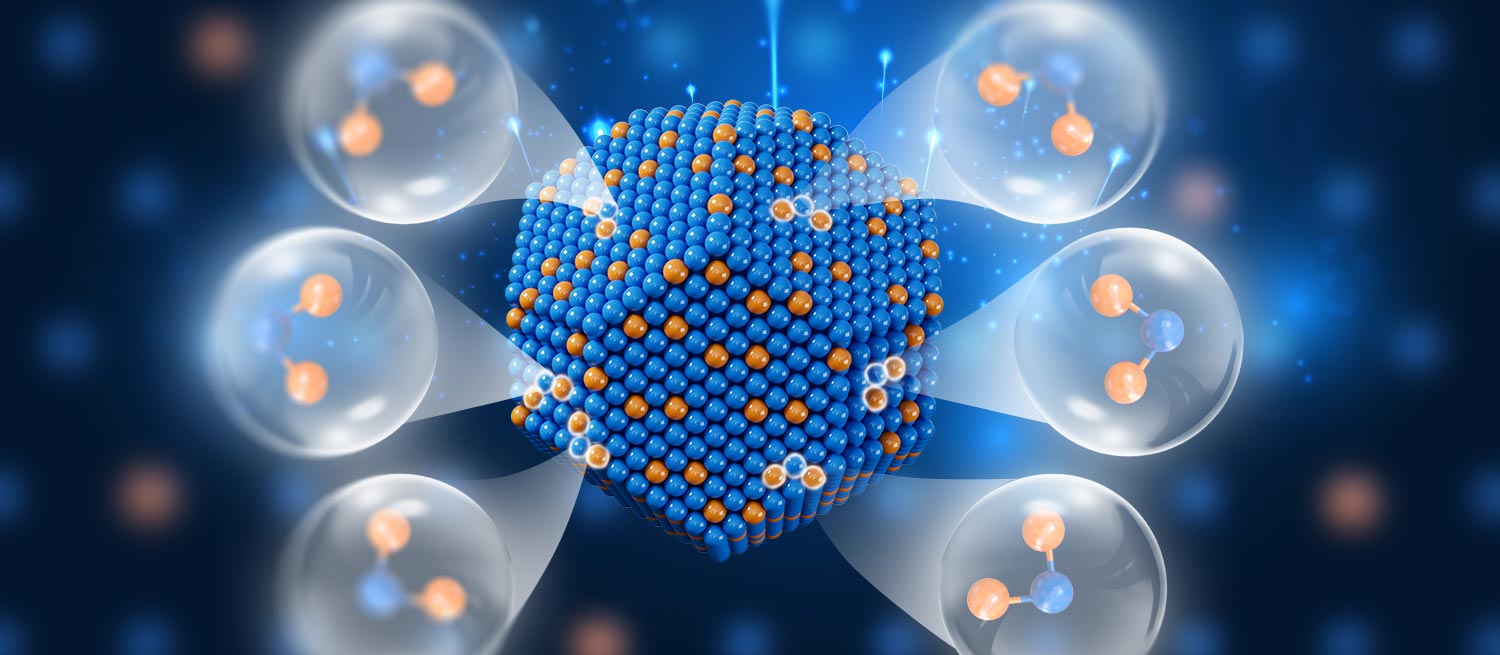
A research team led by Professors Wenjie Shen and Yong Li from the Dalian Institute of Chemical Physics has achieved atomic precision in tuning bimetallic catalyst active sites, enhancing hydrogenation selectivity and activity. By constructing Pt-Fe-Pt heterotrimers on α-Fe nanoparticles through H2 reduction, they improved the hydrogenation rate of crotonaldehyde by 35 times. This innovation addresses the long-standing activity-selectivity trade-off associated with catalytic reactions and was detailed in the journal Chem. Bimetallic catalysts, featuring a noble metal combined with a base metal, offer unique properties for selective heterogeneous hydrogenation, informed by their geometric and electronic structures.
The scientists focused on nanoscale engineering, which optimizes the dispersion of noble metal atoms and impacts electronic structures at an atomic level, fine-tuning catalytic performance. They developed a method to achieve a precise arrangement of isolated Pt atoms into heterotrimers on iron nanoparticles. The resulting configuration demonstrated a preference for hydrogenating the C=O bond over the C=C bond in crotonaldehyde, resulting in enhanced reaction rates. This research not only provides molecular-level insights into catalytic functions but also presents a strategy for designing bimetallic catalysts with atomic precision, offering significant implications for future catalytic applications.